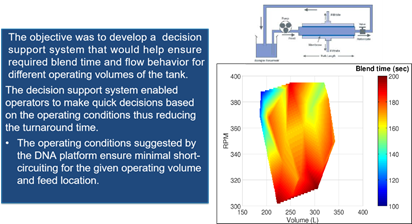
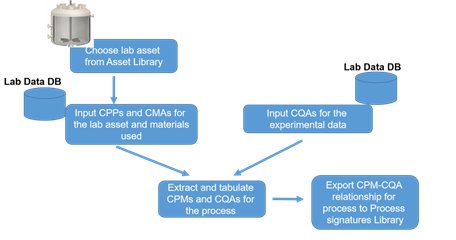
Bioreactor Characterization – Asset Library
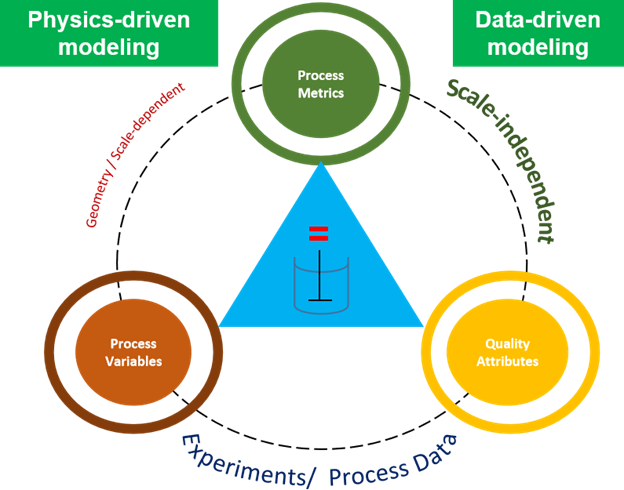
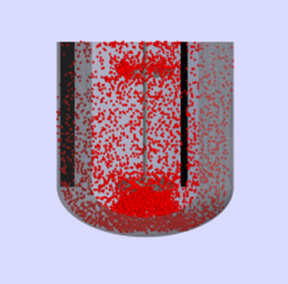
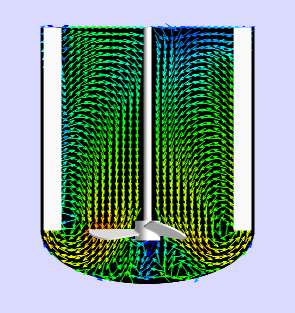
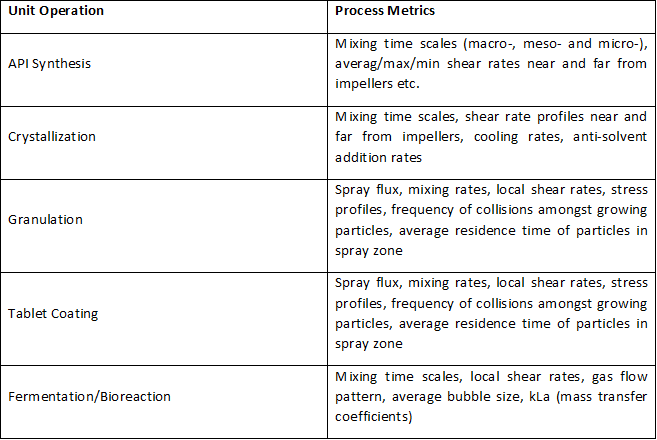
Before we get into what’s the need for an Asset Library, let’s revisit the importance of Asset Characterization in technology transfer and Scale Up Process! Asset Characterization is an important step in determining the process performance capability of an equipment. Using Computational Tools / Software to characterize assets has reduced considerable time in planning future projects. Asset […]