Process signatures capture the scale-independent relationships for a given unit operation or unit process. They represent scale-up or transfer rules that can be used to seamlessly move processes from one equipment to another, from site to another etc. Process signatures are based on the tenet that achieving process similarity is the same as achieving similarity in the processing environment (inside equipment). In our parlance, it means that maintaining Critical Process Metrics the same between scales/equipment/sites is sufficient for successful process transfer. Process signatures build on the notion of process similarity to provide a more holistic solution to process transfer. As noted in a previous post, process signatures relate critical process metrics (CPMs) to critical quality attributes (CQAs) thus providing a systematic, quantitative process transfer workflow. Conceptually, this workflow consists of the following steps:
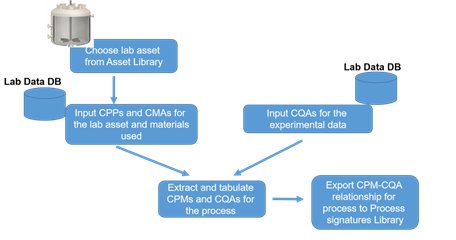
- Collect process data from laboratory, pilot-scale and commercial scale equipment as needed/available. Process data typically is in the form of CPPs, CMAs and CQAs. In addition, the geometry/scale details of the equipment will need to be collected as well.
- Using the DNA methodology of asset characterization, develop relations between CPPs and CPMs for each equipment used to generate the process data.
- Use data-driven methods – like response surface methods (RSM) or multivariate statistical analysis (MVA) – to determine relationships between CPMs and CQAs. Since CPMs represent the internal environment of a process equipment, these relationships are largely scale independent. The adjoining schematic shows the workflow in the form of a flowchart.
- When initiating a process transfer, use these CPM-CQA relationships along with the DNA information for the target equipment (at the receiving unit) – i.e., CPP-CPM relationships – to determine the appropriate CPP value ranges that will ensure acceptable CQAs.
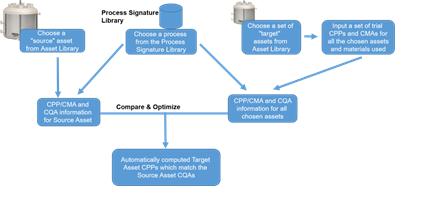
Describing CQAs’ dependences explicitly in terms of CPMs is also advantageous from a process control perspective. Knowing (quantitatively) how the internal environment of a process equipment impacts quality attributes, we can devise control strategies to manipulate process performance. This can be quite empowering to an engineering team during technology transfer. The sending and receiving units (SU and RU respectively), can communicate entirely in terms of CQA-CPM relationships. For instance, the SU can specify a certain combination of kLa, mixing time and average gas bubble size ranges as representing the best processing environment for good product quality (titer value for instance). The RU can use this information in conjunction with their DNA database to determine the appropriate CPPs for the equipment at their facilities. In this way, facility fit assessments can be performed in a meaningful and reliable manner.
Scaleup and technology transfer, when performed in the manner described above, allow for systematic knowledge transfer between the SU and the RU. Because process understanding provides a sound basis for all activities, there is less fire-fighting and more control over quality during process transfer. With an approach like this, one can imagine technology transfer becoming more of something to look forward to – because things get done right first time, all the time!
– Mothivel Mummudi Boopathy

