Process metrics or PMs, are characteristics of a pharmaceutical process that are critical to ensuring product quality and process performance. They are characteristics of the processing ‘micro-environment’ for a given unit operations equipment or ‘process asset’. Generally speaking, maintaining these CPMs at the same (or nearly same) values across scales is a necessary condition for maintaining product quality and process performance during scale-up or scale-down. We can further classify PMs into Critical Process Metrics (CPMs) which influence the product quality attributes (such as particle size distributions, titer values etc.) and Key Process Metrics (KPMs) which influence process performance attributes (such as yield, selectivity etc.). Identifying the appropriate set of CPMs and KPMs for a given unit operation therefore becomes an important first step towards achieving reliable control on critical product quality attributes as well as ensuring economically beneficial process performance.
Some examples of PMs for unit operations are shown in the table below:
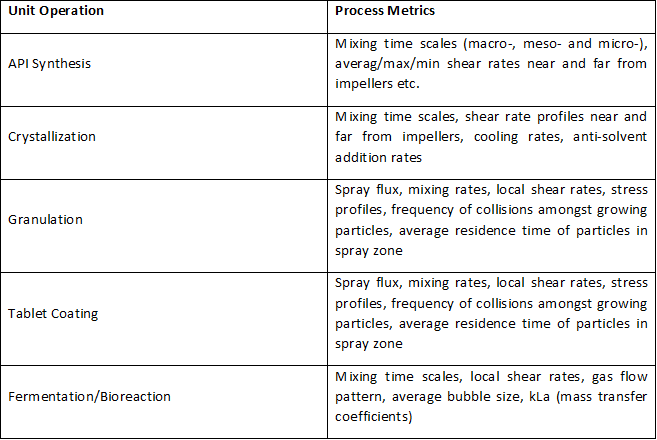
One of the things that can be easily noted about PMs is that they are usually not directly measurable and have to be estimated/calculated. Further, PMs are usually related to the flow environment (which in turn depends on equipment geometry) existing within the process asset and due to this dependence, the so-called ‘transport effects’ come into play during scale or equipment transfer.
In the past, before computational modeling became a viable means for determining flow patterns and transport effects, generic correlations were used to estimate some (but not all) relevant PMs. It was clearly recognized even then, that correlations fell short in terms of satisfying the need to reliably determine asset-specific, ‘geometry-aware’ values for PMs.
With the advent of computational flow modeling for fluids (CFD) and solids (DEM), the estimation of PMs that are relevant to a process is a relatively straightforward exercise. In fact, using a fairly well-established methodology, a comprehensive characterization of a process asset can be performed with a very small resource investment. The returns on this investment are well worth the effort. With a well characterized process asset, engineering teams tasked with process transfer can reliably and confidently
- communicate the process requirements in terms of PMs,
- perform process fit calculations and facility gap analyses in a more meaningful manner
- understand and evaluate the risk involved in choosing assets for campaigns
to name a few obvious use cases. Process metrics are fundamental to process understanding; deploying systematic methods to identify and determine PMs is an important first step towards achieving reliable and risk-free process technology transfer.
– Mothivel Mummudi Boopathy

