Blogs

The Power of Simulations: How to Harness Data for Informed Decision-Making
In the dynamic landscape of decision-making, data takes center stage. However, challenges such as incomplete data, prolonged decision timelines, and resource loss due to delays
Bioreactor Characterization – Asset Library
Before we get into what’s the need for an Asset Library, let’s revisit the importance of Asset Characterization in technology transfer and Scale Up Process! Asset

Asset Characterization with Interactive Webapps
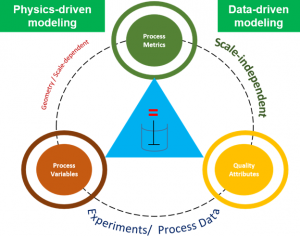
Webapps are a great tool to create an interactive interface to visualizing the interaction of Process Parameters (PP), Process Metrics (PM) and Quality Attributes (QA).
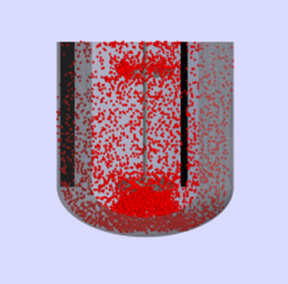
Scale-up of Bioreactors: Revisiting the Heuristics
“It is paradoxical, yet true, to say, that the more we know, the more ignorant we become in the absolute sense, for it is only
What exactly is Feature Engineering ?
The goal is to turn data into information, and information into insight. – Carly Fiorina, Ex – C.E.O Helwett-Packard What exactly is Feature Engineering ?

Simple webapps for Fundamental Process Understanding
Ever wished for a way of easy dashboard reporting of advanced analysis across the organization with interactive abilities? Simple webapps are the perfect solution to
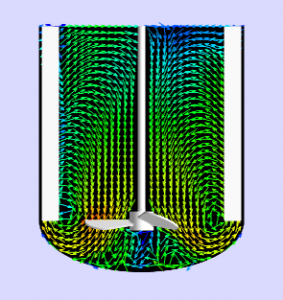
Scale Up of Bioreactors – Have you ‘Understood’ your asset yet?
“I believe that thirty million of these animalcules together would not take up as much room, or be as big, as a coarse grain of
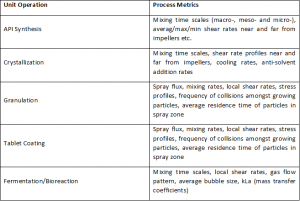
Process Metrics: The Keystone of Process Understanding
Process metrics or PMs, are characteristics of a pharmaceutical process that are critical to ensuring product quality and process performance. They are characteristics of the
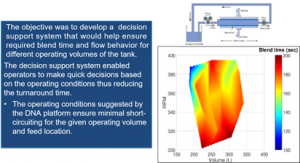
Asset Characterization: Digital Nameplating of Assets
Asset characterization is an important step in determining the process performance capability of a given unit operations equipment. As we saw in an earlier post,
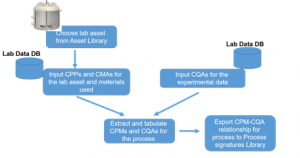
Process Signatures: How can you stop worrying & start loving Scale Up
Process signatures capture the scale-independent relationships for a given unit operation or unit process. They represent scale-up or transfer rules that can be used to

